French title: Performance du taux d'interleukine 18 (IL-18) sérique pour la surveillance des patients atteints de fièvre méditerranéenne familiale.
First author: Inès Elhani
Journal: The Journal of Allergy and Clinical Immunology: In Practice (JACIP)
Author of the abstract: https://pubmed.ncbi.nlm.nih.gov/39667435/
Article traduit par le Dr Catherine Grandpeix-Guyodo

Introduction:
Inflammasome activation in Familial Mediterranean Fever (FMF) leads to increased secretion of interleukin (IL)-1β and IL-18. Monitoring FMF activity is essential due to the risk of AA amyloidosis in cases of prolonged inflammation and is classically done using CRP and SAA (serum amyloid A protein), whose values may be dissociated. This study investigated the possibility of monitoring FMF activity through total blood IL-18 assay.
Patients and methods:
This monocentric, retrospective study involved adult FMF patients who had at least one total blood IL-18 assay during their follow-up between 2022 and 2024. The data collected included the mutational status of the MEFV gene, CRP and SAA values, disease activity (considered controlled if fewer than 2 flares per year / uncontrolled if 2 or more flares per year), and finally the total IL-18 assay(s) performed during follow-up consultations (routine care).
Results:
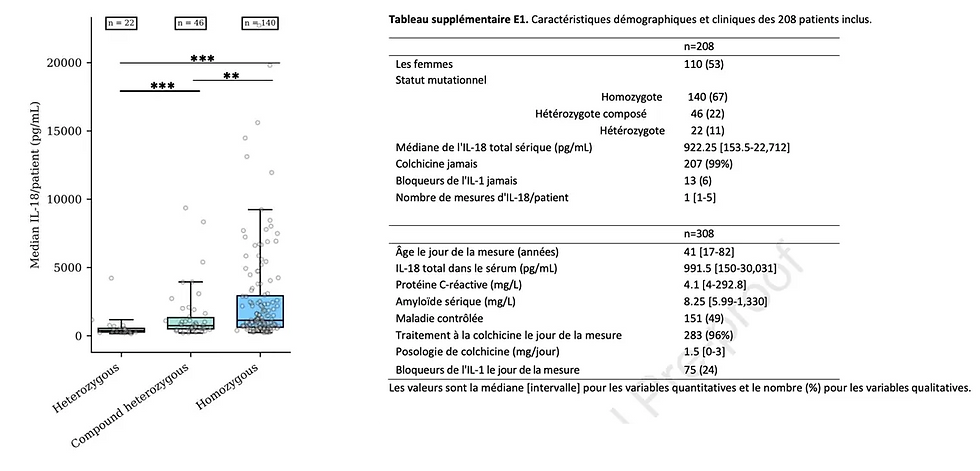
Among 208 sampled patients, half had controlled FMF, and a total of 308 IL-18 assays were analyzable with a median measurement of 922.25 pg/mL (N < 350 pg/mL). Among patients with controlled FMF, IL-18 levels were significantly higher in homozygous patients compared to compound heterozygotes and heterozygotes.
Some patients had IL-18 assays when FMF was inactive and active, and levels showed no significant difference.
IL-18 levels were not significantly different in patients treated with anti-IL-1.
Assays > 7,000 pg/mL concerned 16 patients who had adherence issues with their colchicine treatment and rather low dosages (< 2 mg).
Discussion:
Total blood IL-18 levels appear to be correlated with genotype but not with disease activity. The persistence of high IL-18 levels in asymptomatic patients could suggest low-grade activity of the pyrin inflammasome. Very high levels may show that patients are undertreated, but the significance of IL-18 levels in terms of amyloidosis risk remains to be determined if the SAA level is normal.
The limitations of this study are the few samples per patient (generally 1), the retrospective nature, and the absence of evaluation by a disease activity score at the time of sampling.
Conclusion:
The monitoring of total blood IL-18 levels has a role that remains to be defined since it does not seem to reflect either the patient's immediate inflammatory state or FMF activity. Its interest could lie in detecting subclinical inflammatory activity and evaluating treatment adherence. Prospective studies on large cohorts will be necessary to deepen its utility in FMF.